Fluorescence recovery after, and loss in, photobleaching (FRAP and FLIP)By splitting the excitation laser light of our microscope on the basis of polarization we can generate two independent paths , one of which can be utilised for TIRF and widefield epifluorescence illumination, the other for focussed-light studies, in effect generating a diffraction-limited spot in the plane of the sample.
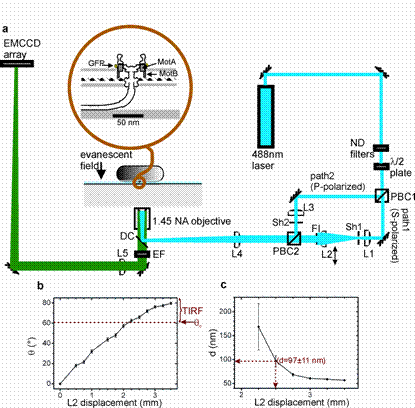
a, Schematic of TIRF microscope (ND=neutral density filters, PBC=polarizing beam-cube, L=lens, DC=dichroic mirror, EF=emission filter,FI=field iris, Sh=shutter). b, Variation of emergent angle q from objective lens with lens L2 displacement from the optic axis. c, Equivalent variation of depth of penetration d of the evanescent field. This focussed spot can generate a highly intense and localized excitation field of typically ~1 micron diameter which can be utilized to photobleach fluorescently-tagged proteins i a specific sub-region of a biological cell e.g. one of the poles of the bacterium Escherichia coli. We can then monitor recovery of fluorescence intensity into this dark bleached region (FRAP), and in many cases in bleaching this background fluorescence the imaging contrast will have improved sufficiently to permit single-particle tracking of diffusing components, down to the level of single fluorophore molecules, whose precise position can then be estimated using so-called fluorescence imaging with one nanometre accuracy (FIONA). Similarly, we can observe decreases in fluorescence intensity in areas surrounding the initial focussed-spot bleach zone, in effect "single-shot" fluorescence loss in photobleaching (FLIP).
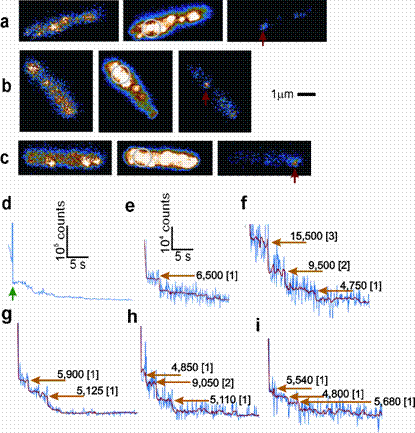
Less noisy steps obtained by pre-bleaching. a‑c, Three example cells: left panel=pre-bleach, middle panel=focussed laser bleach (circle=extent of original laser focus width), right panel=immediately post-bleach. Position of motor under observation indicated (red arrow). d, A typical trajectory of the motor component of fluorescence intensity, with the focussed laser spot pre-bleach indicated (green arrow). e-i, Expanded data from several motors in the post-bleach region showing raw motor intensity (blue), Chung-Kennedy filtered motor intensity (red) and position of detected steps (orange arrow) with the measured step size for each indicated along with [equivalent corresponding number of bleached GFP molecules to the nearest integer] based on a unitary step size of ~5,400 counts s-1. FRAP and FLIP data may be modelled by e.g. Monte Carlo simulations, in which the known geometry of the biological cell is utilized to predict the movement of several diffusing molecules either in the 3D interior of the cell (in the so-called cytoplasm) or over its 2D surface in one of the cell membranes. In doing so we impose trial values of diffusion coefficient, and such predictions can then be compared with experimental FRAP and FLIP data to estimate the experimental rate of diffusion.
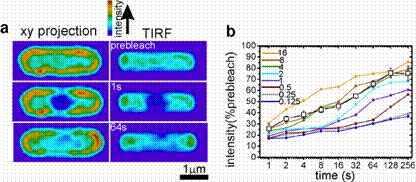
Estimating the diffusion coefficient in the membrane by comparison with simulation. a, x-y projections and TIRF images (averages of 10 simulations) before and after focussed bleaching of the centre of the cell, D=1×10‑3 mm2 s‑1. b, Intensity in ROI centred on laser focus vs. time postbleach. Simulated traces (coloured) for values of D in range (0.125 to 16)×10‑3 mm2 s‑1 and experimental data (black squares) from FRAP in non-motor regions (averaged from 8 GFP‑MotB cells, error bars one s.d.) |
|
 |
DHTML and javascript menus courtesy of
TwinHelix Designs
maintained by Mark Leake last updated: 17 February 2013 |